
Biogen is conducting a clinical trial to evaluate the efficacy and safety and of an investigational drug for adults who do not have any clinical signs or symptoms of Amyotrophic Lateral Sclerosis (ALS) that definitely indicate the onset of ALS, but do carry a certain superoxide dismutase 1 (SOD1) gene variant.
Study duration
The maximum study duration is approximately six years and six months, including:
- Up to 6 weeks screening
- Up to 4 years and 3 months health monitoring
- Up to 2 years study treatment period
The study is comprised of 4 parts as described below.
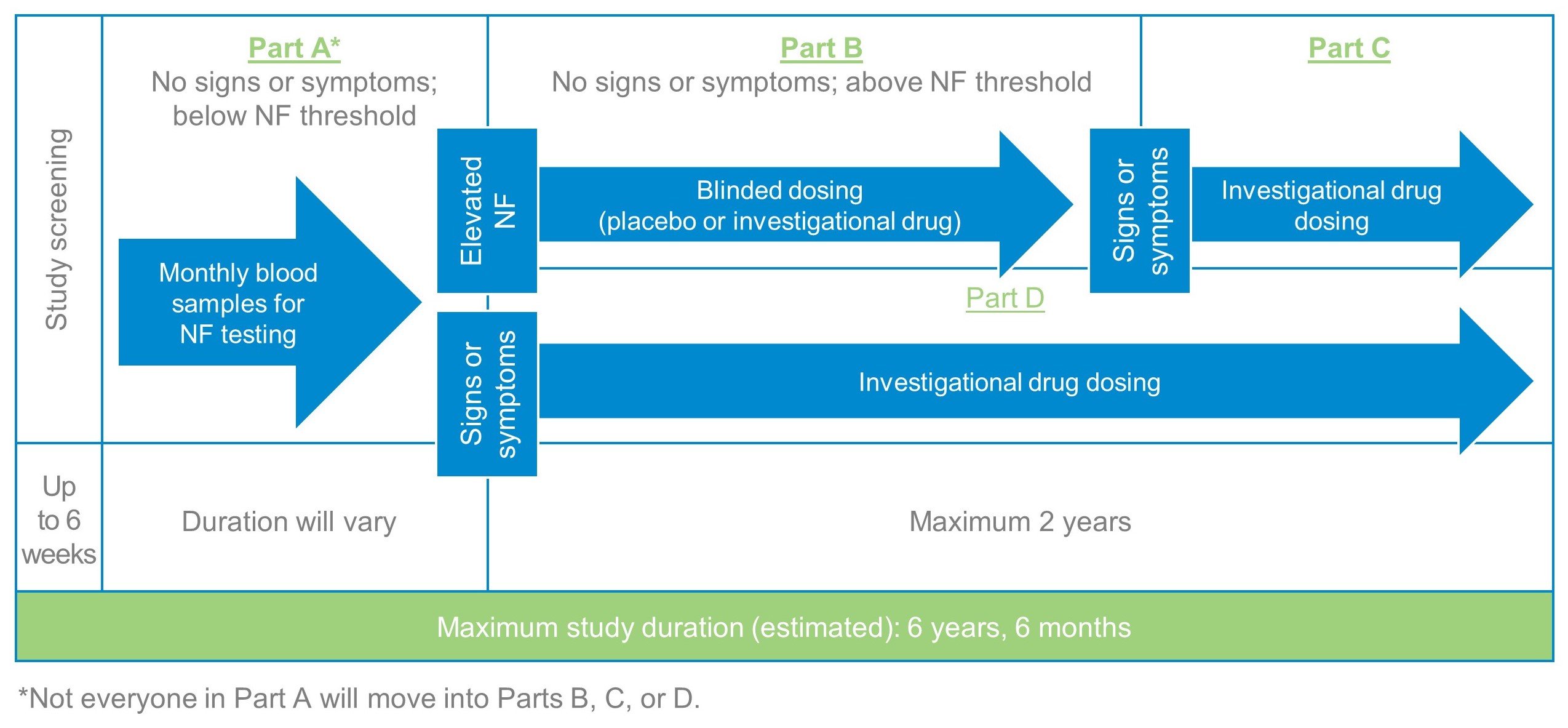
NF= Neurofiliment which is a group of proteins.
Investigational drug
- Tofersen (BIIB067), an antisense oligonucleotide (ASO)
- Designed to reduce the amount of SOD1 protein in the body
- Given intrathecally, through a lumbar puncture
Key Eligibility Criteria
To be able to enroll in the ATLAS study (Part A), participants need to:
- Not have any signs or symptoms of ALS (that definitely indicate onset of ALS)
- Have a certain SOD1 mutation (that is associated with high/complete penetrance and rapid disease progression)
- Be 18 years of age or older
- Have a plasma NF level below the pre-defined threshold
All eligible participants will receive at no cost
- Comprehensive study-related health evaluations and assessments, including genetic testing
- Investigational drug or placebo
- All study-related visits and care
Assistance with travel and accommodations, and reimbursement for study-related expenses may also be available.
Read more on the ATLAS trial here or reach out to atlas@tricals.org for more information.
Related news

Meet the centre - Torino ALS Center (University of Torino)

Amylyx withdraws RELYVRIO/AMX0035 from U.S. and Canadian markets

Results of TUDCA-study: No effect of investigated compound
