
In the run-up to the virtual 31st International Symposium on ALS/MND, the ENCALS Satellite meeting was held online this year. The goal of this meeting is to discuss international collaborations, SOPs and translation of new developments to the clinic. Nearly 200 people attended this year.
 A brief overview of the recent developments of TRICALS and the ENCALS network was given by chairman Prof Leonard van den Berg (UMC Utrecht, the Netherlands). He discussed phase 3 trials in which TRICALS is currently involved and the preparation of several investigator-initiated trials, such as the MAGNET study and the Lighthouse 2 trial.
A brief overview of the recent developments of TRICALS and the ENCALS network was given by chairman Prof Leonard van den Berg (UMC Utrecht, the Netherlands). He discussed phase 3 trials in which TRICALS is currently involved and the preparation of several investigator-initiated trials, such as the MAGNET study and the Lighthouse 2 trial.
 As the live ENCALS meeting in June was cancelled, Professor Ammar Al-Chalabi (King’s College London, UK) presented the ENCALS Young Investigator award virtually during the meeting. This prize recognises the brightest and best young scientists in ALS, and is given for outstanding research that is innovative, challenges existing ideas about ALS, results with patient benefit, and impacts on our understanding of ALS. Dr. Tatyana Shelkovnikova (Cardiff University, UK) won the award for her impressive research on RNA metabolism.
As the live ENCALS meeting in June was cancelled, Professor Ammar Al-Chalabi (King’s College London, UK) presented the ENCALS Young Investigator award virtually during the meeting. This prize recognises the brightest and best young scientists in ALS, and is given for outstanding research that is innovative, challenges existing ideas about ALS, results with patient benefit, and impacts on our understanding of ALS. Dr. Tatyana Shelkovnikova (Cardiff University, UK) won the award for her impressive research on RNA metabolism.
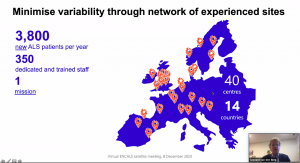
 Professor Orla Hardiman (Trinity College Dublin, Ireland) provided an excellent overview of the TRICALS ICT Platform that aims to serve as a digital umbrella linking all ENCALS registered sites (currently 65). The goal is to establish a pan-European ALS registry that combines all available data ever collected from patients. The TRICALS ICT Platform could be the next step in analysing ALS subgroups, identifying the best treatment options, enhancing clinical trials globally and providing a harmonising framework for all stakeholders.
Professor Orla Hardiman (Trinity College Dublin, Ireland) provided an excellent overview of the TRICALS ICT Platform that aims to serve as a digital umbrella linking all ENCALS registered sites (currently 65). The goal is to establish a pan-European ALS registry that combines all available data ever collected from patients. The TRICALS ICT Platform could be the next step in analysing ALS subgroups, identifying the best treatment options, enhancing clinical trials globally and providing a harmonising framework for all stakeholders.
Closely related was the talk by Dr Julian Grosskreutz (Friedrich Schiller University Jena, Germany). He revealed how predictive modelling strategies could help to classify patients in terms of disease progression based on advanced data obtained from wet biomarkers (e.g. obtained from blood), imaging or electrophysiology.
During the COVID-19 pandemic, clinical research in ALS had come to a hold. Not only are patients living with ALS a high-risk group, also some of the vital clinical measurements such as spirometry (lung function) were impossible to obtain. Professor Adriano Chiò (University of Turin, Italy) showed how markers in the blood could reveal reliable insights into the respiratory function of patients. Especially when routine spirometry cannot be performed, the blood gas analysis could serve as a proxy and can be easily obtained.
 At last, there was a lively discussion about the need for pharmaceutical companies to conduct clinical trials in paediatric ALS (i.e. ALS before the age of 18), led by TRICALS researcher Tessa Kliest (UMC Utrecht, the Netherlands). TRICALS investigated the possibilities and requirements to conduct paediatric trials in these populations and summarised the overall opinion among European investigators. The results will be published soon as a TRICALS-ENCALS collaborative work in a peer-reviewed journal.
At last, there was a lively discussion about the need for pharmaceutical companies to conduct clinical trials in paediatric ALS (i.e. ALS before the age of 18), led by TRICALS researcher Tessa Kliest (UMC Utrecht, the Netherlands). TRICALS investigated the possibilities and requirements to conduct paediatric trials in these populations and summarised the overall opinion among European investigators. The results will be published soon as a TRICALS-ENCALS collaborative work in a peer-reviewed journal.
Related news

Meet the centre - Torino ALS Center (University of Torino)

Amylyx withdraws RELYVRIO/AMX0035 from U.S. and Canadian markets

Results of TUDCA-study: No effect of investigated compound
