Investigational drug
Approximately 2% of people living with ALS have a mutation in the SOD1 gene. Mutations in the SOD1 gene can lead to the production of abnormal SOD1 protein that is likely to be toxic to cells and could possibly lead to nerve cell death observed in people with ALS. The ATLAS study is evaluating whether an investigational drug (tofersen) may delay the onset of signs or symptoms of ALS and/or slow the decline in function once clinical signs or symptoms appear (compared to the initiation of tofersen at the time of, or after, emergence of ALS)
Tofersen (BIIB067) is an antisense oligonucleotide (ASO), which is designed to reduce levels of SOD1 protein in people with ALS associated with a SOD1 gene variant.
Study overview
The study is comprised of 4 parts as described below.
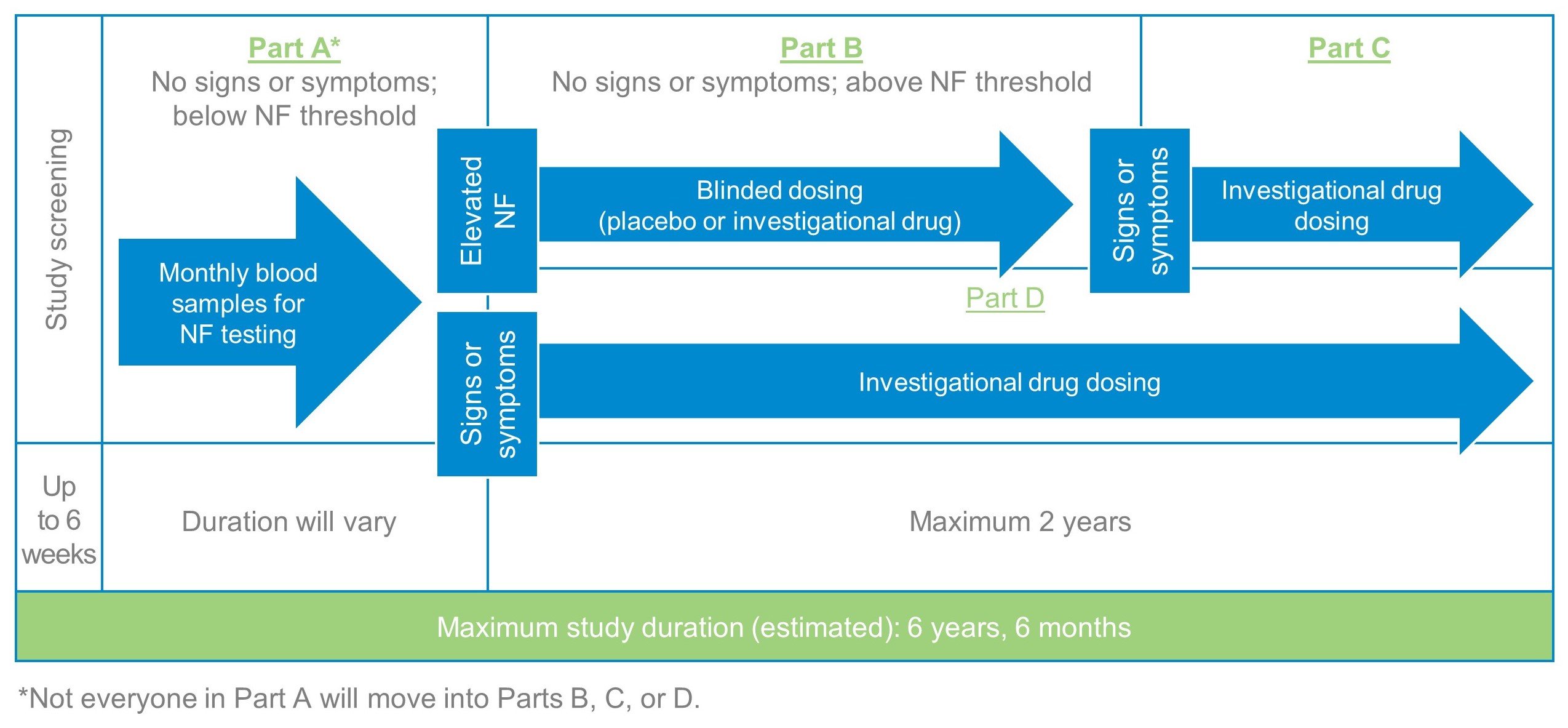
Part A
If you test positive for one of the SOD1 gene variants being studied and pass other screening tests, you will be eligible to enter Part A of the study. During this part, you will not receive any study treatment, but your health status and neurofilament (NF) level will be closely monitored (at home or at the study center). The study team will evaluate your blood samples monthly for an NF level that is above the threshold. You and the study doctor will know when the NF level is above the threshold to consider eligibility for the next part of the study (Part B). The duration of Part A will vary.
Part B
If your NF level increases above the pre-determined threshold during your participation in Part A, the study doctor will evaluate the cause of the increase in NF. If the study doctor determines that the NF increase is not attributable to an alternative cause unrelated to ALS disease activity, you may choose to be screened for eligibility for potential participation in Part B. During this part of the study, you will be assigned to receive either the investigational drug or a placebo. You will be assigned randomly by a computer, and you will have an equal chance of receiving the investigational drug or placebo. Neither you nor the study team will know whether you received the placebo or the investigational drug; this is called a double-blind period.
The investigational drug or placebo is delivered intrathecally. Intrathecally means that the study drug is given to you by a procedure called a lumbar puncture.
Part C
If you show signs or symptoms of ALS during Part B of the study and your study doctor and a group of independent reviewers confirm that these signs or symptoms definitely indicate onset of ALS, you may choose to be screened for eligibility for potential participation in Part C of the study. During this part, everyone will receive the investigational drug; this is called an open-label period. The total length of participation in Parts B and C of the study is up to approximately two years.
Part D
If you start to show signs or symptoms of ALS during your participation in Part A or during screening for Part B, and your study doctor and a group of independent reviewers confirm that these signs or symptoms definitely indicate onset of ALS, you may choose to be screened for eligibility for potential participation in Part D of the study. Part D is another open-label period during which everyone will receive the investigational drug. The total length of participation in Part D is up to approximately two years.
Key Eligibility Criteria
To be able to enroll in the ATLAS study (Part A), participants need to:
- Not have any signs or symptoms of ALS (that definitely indicate onset of ALS)
- Have a certain SOD1 mutation (that is associated with high/complete penetrance and rapid disease progression)
- Be 18 years of age or older
- Have a plasma NF level below the pre-defined threshold
If you are eligible and choose to participate, you will receive the following at no cost:
- Comprehensive study-related health evaluations and assessments, including genetic testing
- Investigational drug or placebo
- All study-related visits and care
Travel Support
Assistance with travel and accommodations, and reimbursement for study-related expenses may also be available.




